Developing life more humann
We’re focused on creating the cutting-edge therapies and technologies that can change life for the better.
Our pipeline reflects how we, as well as our partners, are applying MannKind technology to develop and advance new products to not only help people take control of their health, but truly thrive.
 Cardiometabolic
Cardiometabolic
 Orphan Lung
Orphan Lung
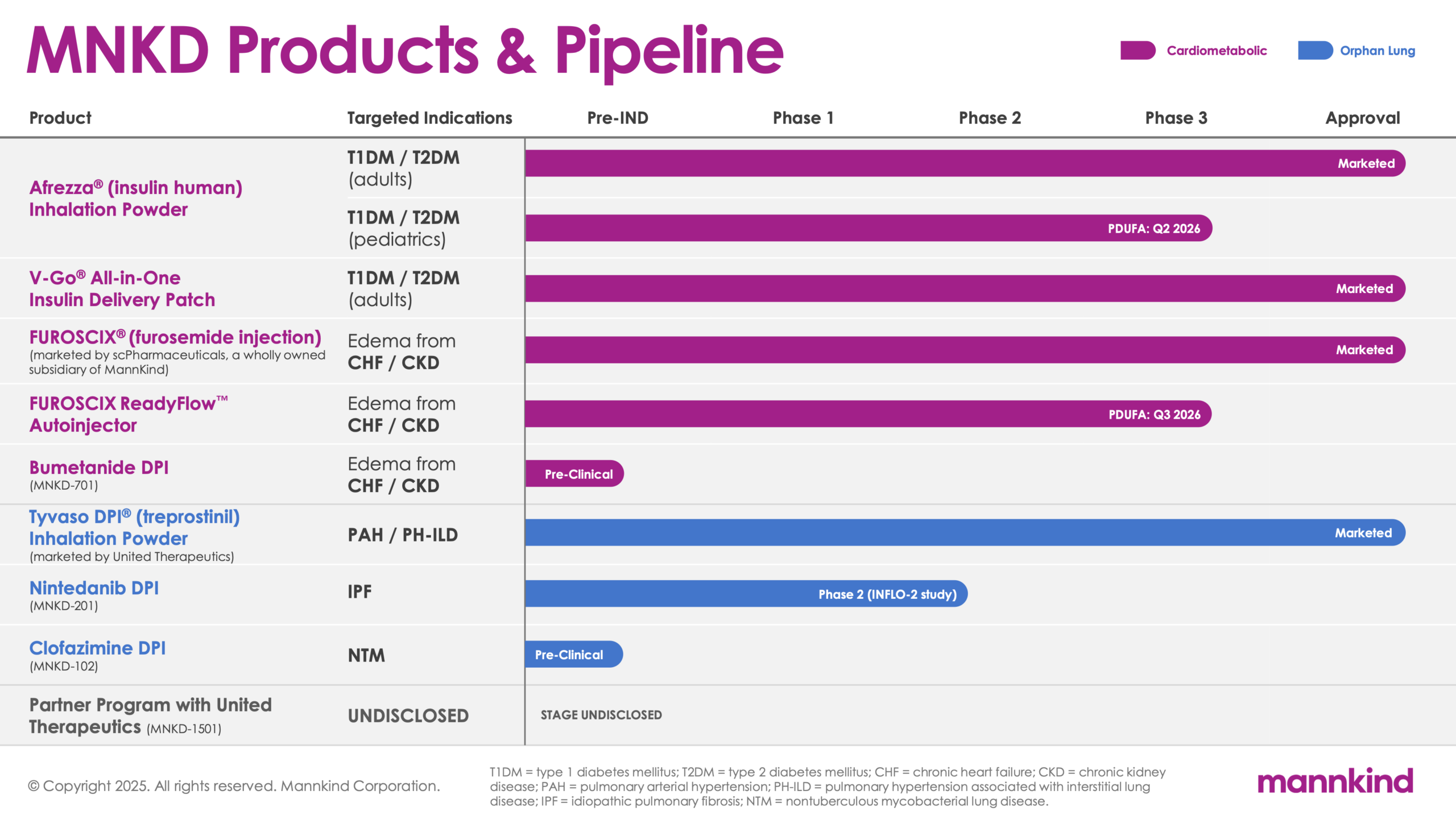
| Product | Targeted Indications | Pre-IND | Phase 1 | Phase 2 | Phase 3 | Approval |
|---|---|---|---|---|---|---|
Afrezza® (insulin human) Inhalation
Powder

|
|
|
||||
V-Go® All-in-One Insulin Delivery Patch

|
|
|
||||
|
FUROSCIX® (furosemide injection) (Marketed by scPharmaceuticals, a wholly owned subsidiary of MannKind) 
|
|
|
||||
| FUROSCIX ReadyFlow™ Autoinjector |
|
|
||||
|
Bumetanide DPI (MNKD – 701) |
|
|
||||
|
Tyvaso DPI® (treprostinil) Inhalation Powder (marketed by United Therapeutics) 
|
|
|
||||
|
Nintedanib DPI (MNKD – 201) |
|
|
||||
|
Clofazimine DPI (MNKD – 102) |
|
|
||||
| Partner Program with United Therapeutics (MNKD – 1501) |
|
Stage Undisclosed | ||||
|
T1DMType 1 Diabetes Mellitus T2DMType 2 Diabetes Mellitus CKDChronic Kidney Disease |
PAHPulmonary Arterial Hypertension PH-ILDPulmonary Hypertension Associated with Interstitial Lung Disease CHFChronic Heart Failure |
IPFIdiopathic Pulmonary Fibrosis NTMNontuberculous Mycobacterial Lung Disease |