-
250M
CARTRIDGES/YR IN CAPACITY
-
>40
MOLECULES FORMULATED
-
ISO 8
PRODUCTION CLEAN ROOMS
-
328K
TOTAL SQUARE FEET
Bring your vision to market
As a fully licensed drug and medical device manufacturer, we have the space, equipment, quality control, and expertise to make technologies and therapies that can change lives for the better.
Our pipeline reflects how we, as well as our partners, are applying MannKind technology to develop and advance new products to not only help people take control of their health, but truly thrive.
Our capabilities:
At our FDA-inspected and approved site, we develop advanced drug delivery systems that use our breakthrough technology.
-

Lyophilization and spray-drying production
-

Small molecule, peptide, and protein experience
-

Manufacturing insight and optimization
-

Formulation development personnel and laboratory space
-
Device development personnel and laboratory space
-
Packaging development
-

Pilot chemical and formulation plant
-

Clinical manufacturing and packaging suites
-

Support services, including quality, analytics and in-house microbiology support
-

come partner with us
Technosphere® Technology
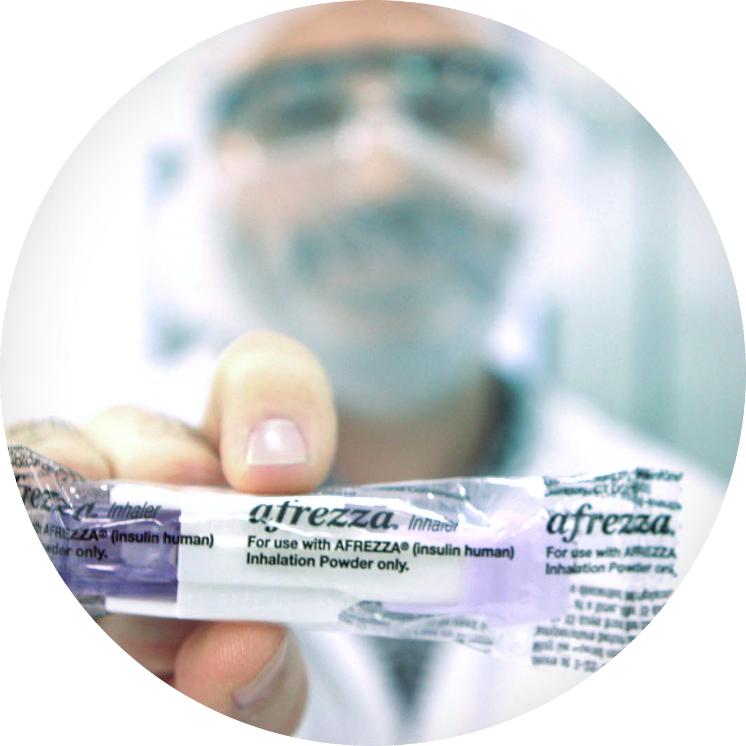
State-of-the-art development technologies optimize dry powder formulation with inhalation devices early in the development process to ensure successful drug delivery in first-in-human studies.
- Molecular weight of 200 to 150,000 daltons (Da)
- Drug content up to 90% by weight
- Perfectly sized for inhalation
- Fixed in size prior to API introduction
- Free from requiring sizing, milling or blending
Inhalation innovation
Small, portable, and easy to use
-

DREAMBOAT® Licensing Platform
Re-usable Dreamboat (Gen 2) FamilyFor chronic therapies
-

CRICKET® Licensing Platform
Single use Cricket Solutions
For more acute therapies
The breath-powered inhalers from MannKind, which are available for collaboration projects or licensing, are simple, easy to use and discreet. They are disposable, and no cleaning is required. More importantly, they have excellent delivery efficiency.
Applicable to a broad range
of therapies and formulations
Wide achievable dose range
0.001 mg to 5 mg from a single cartridge
Proven to deliver
up to 70% of the dry-powder dose deep into the lungs
Flexible for use
with small-, medium-, or large-dose cartridges
Manufactured readily
at a low cost

BluHale® Inhalation Training Device
BluHale helps make our simple inhaler even easier to use. The BluHale connected care ecosystem includes:
-
BluHale PRO® edition
For healthcare providers for in-office training on effective inhalation techniques
-
BluHale VIS® edition
For consumers has additional sensors to detect device orientation plus dosage strength and timing
(Currently under development)
h-patchTM
The wearable h-Patch drug delivery platform, upon which the V-Go® once-daily wearable insulin delivery device is built, provides a continuous, preset, subcutaneous rate of medication for 1-72 hours via a needle that remains under the skin.
Designed to be patient friendly, the hydraulically engineered device:
-
Is discreet
Worn like a patch
-
Is easy to use
No complicated technology
-
Eliminates injections
No medication by pens or syringes

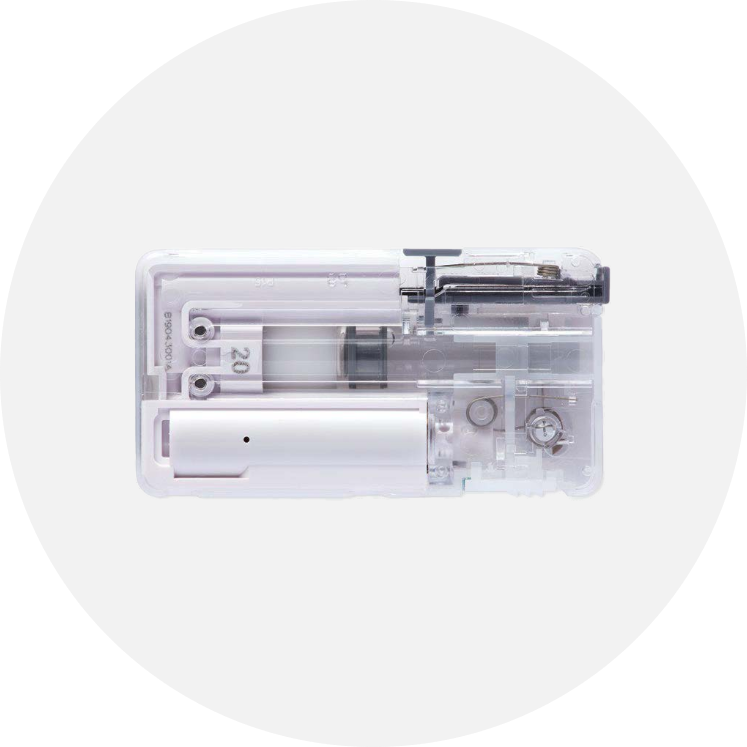
Intellectual property protection is a priority
Technosphere technology
>1,000 patents in place worldwide
>1,000 utility and >200 design patents
The power of patents: Afrezza®
>600 patents provide protection to Afrezza and related technologies.
h-patch:
>150 patents and patent applications*
*INCLUDES WEARABLE H-PATCH DRUG DELIVERY, NEXT GEN DEVICES, METHODS OF USE AND ASSOCIATED TECHNOLOGIES.
SC=SUBCUTANEOUS.
Bring product to market rapidly and responsibly
The MannKind Fast Feasibility program is designed to rapidly obtain technical feasibility and animal proof of concept by:
Preparing Technosphere test powders with API
Timeline: 1-3 months
Deliverables: Assay, drug loading, particle aerodynamics, drug product stability
Evaluating delivery in animal models
Timeline: 1-3 months
Deliverables: PK, bioavailability
Data analysis
Co-author final report
Two ways to connect
Let’s continue the conversation
With our innovative Technosphere and h-Patch technologies, our team of dedicated scientists and medical professionals are developing therapeutic products for people with serious medical conditions to help give them control of their health and live life without limits.
